Pioneering In Vivo CAR T Cell Therapies
Our programs reflect our commitment to improving the treatment experience for patients. Our hope at Umoja is to find in vivo solutions that will fulfill the promise of chimeric antigen receptor (CAR) T cell therapies for every patient in need.
The Promise of CAR T Cell Therapy
The first CAR T cell therapies to become available to patients were highly complex, difficult to scale, and hard to access ex vivo therapies.1-3
Ex vivo CAR T cell therapy = T cells are removed from the body, genetically altered to enhance their effectiveness at fighting against disease (eg, cancer), and then reinfused back into the patient’s body; time from collection of T cells to infusion of ex vivo CAR T cells can take ~3-6 weeks.1,2,4,5
Ex vivo CAR T cell therapies have been shown to be capable of providing high response rates to patients with hematological malignancies, in particular, patients with B cell malignancies in which other therapies have been ineffective (ie, relapsed/refractory [R/R] patients).1,5-10 Systematic reviews and meta-analyses have revealed complete response rates as high as 92%, and overall response rates (ORRs) ranging from 65-85% in R/R hematological malignancies, including B-cell non-Hodgkin lymphoma (B-NHL) and multiple myeloma (MM).10-14 Today, several ex vivo CAR T cell therapies are currently FDA-approved for various types of relapsed or refractory hematological malignancies.6
Current Challenges Facing Ex Vivo CAR T Cell Therapy
Despite the potential benefit that ex vivo CAR T cell therapy might provide to patients, many eligible patients are not receiving this treatment option.2 The Center for International Blood and Marrow Transplant Research (CIBMTR) reported that, in 2022, only ~3500 first ex vivo CAR T cell infusions were administered in the United States, with the largest number (1747) going to patients with large B-cell lymphoma.15 Therefore, using diffuse large B-cell lymphoma (DLBCL) as an example, if ~10,000 US patients were eligible yearly for CAR T cell therapy, only a fraction (less than 20%) of these eligible patients may actually be treated with ex vivo CAR T cell therapy today.2,15,16
Why are all eligible patients not receiving ex vivo CAR T cell therapy?
There are many factors that are currently limiting the use of ex vivo CAR T cell therapy, including patient-, treatment-, and healthcare professional-related factors.1-3,5-7,10,17-19 Receiving ex vivo CAR T cell therapy is one of the more complex and challenging oncology treatment experiences, requiring a complex, lengthy, and expensive manufacturing process; it includes the need for specialized facilities, cells to be harvested and transported to one of a few manufacturing sites, and significant production time.2-5 Time from referral to infusion of ex vivo CAR T cell therapy can take several months.4 Our goal at Umoja is to reduce nearly all of these barriers with our in vivo programs so that all patients in need can receive this groundbreaking treatment option.

Umoja’s Solution: In Vivo Generation of CAR T Cells Through Our VivoVecTM Platform
Umoja’s flagship platform, VivoVec, enables the in vivo generation of CAR T cells within the patient’s body, bypassing the need for CAR T cell production outside of the body and saving precious time and complexity.19,20
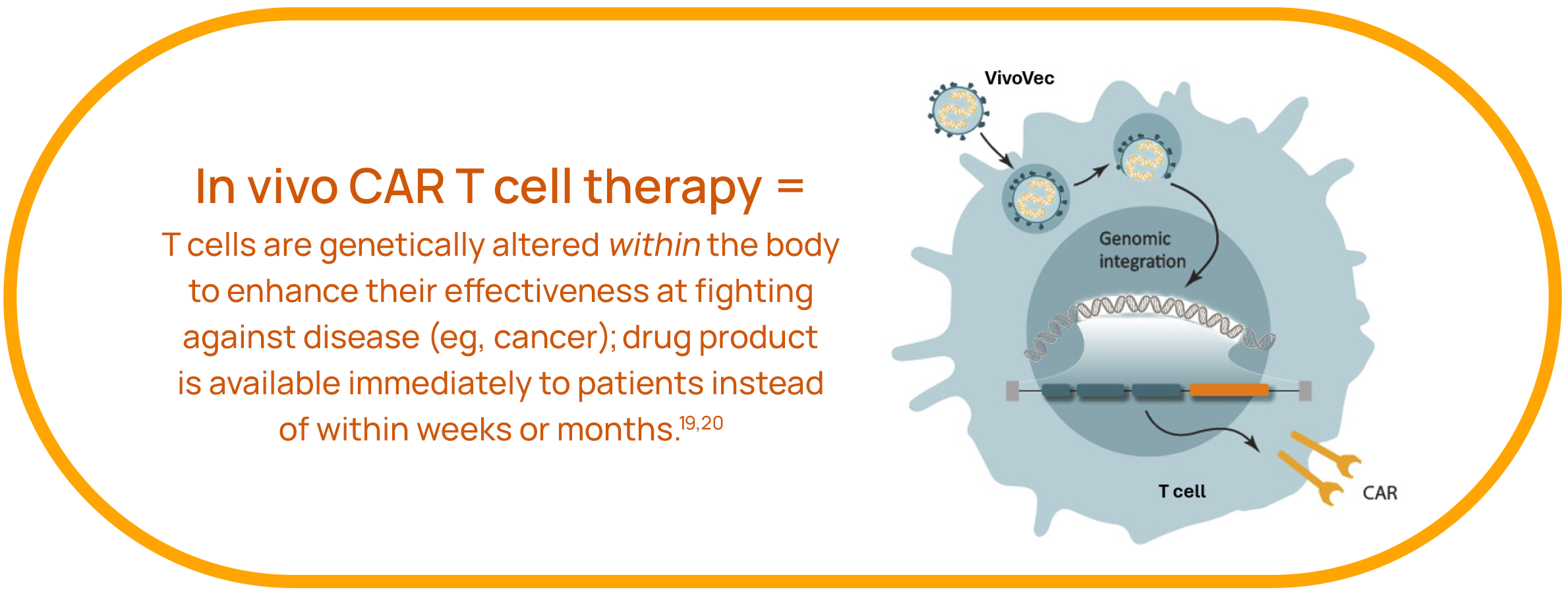
VivoVec aims to simplify, increase the effectiveness, decrease the cost, and speed up the delivery of CAR T cell therapies to patients. Our drug product will be available immediately to patients, removing the typical weeks-to-months-long process associated with today’s ex vivo CAR T cell therapies. With a single infusion of VivoVec, the patient’s body will produce its own CAR T cells. Patients will not need to receive lymphodepleting chemotherapy with VivoVec.
The VivoVec platform utilizes a lentiviral vector delivery system and includes the following unique features.
- Multi-domain fusion (MDF) surface engineering: Our proprietary MDF technology augments VivoVec particles’ binding, activation, and transduction of T cells19
- Cocal fusion glycoprotein pseudotyping: The cocal fusion glycoprotein is resistant to human serum inactivation, enabling VivoVec-based drug products to achieve a high potency19
- Modular and flexible: VivoVec can be used to efficiently deliver any CAR payload, targeting an array of surface targets
- RACRTM platform for enhanced potency: VivoVec can be combined with RACR (rapamycin-activated cytokine receptor) to provide enhanced survival and expansion to VivoVec-engineered immune cells20
Umoja is committed to finding solutions for the current challenges of ex vivo CAR T cell therapy. Other Umoja platforms are under investigation in hematological malignancies and other conditions.20-22
References: 1. Srivastava S, et al. Immunotargets Ther. 2024:13:413-433. 2. Odstrcil MS, et al. Blood Rev. 2024:63:101136. 3. Dias J, et al. Mol Ther Methods Clin Dev. 2024;32(2):101250. 4. Battiwalla M, et al. Blood Adv. 2024. Online ahead of print. 5. Mikhael J, et al. JCO Oncol. Pract. 2022;18(12):800-807. 6. Vera DG, et al. Hum Vaccin Immunother. 2024;20(1):2378543. 7. Olejarz W, et al. Int J Mol Sci. 2024;25(14):7743. 8. Zhou D, et al. Cancer Med. 2024;13(11):e7375. 9. Imai Y. Int J Hematol. 2024;120(1):3-5. 10. Elmarasi M, et al. Int Immunopharmacol. 2024:135:112312. 11. Shahzad M, et al. Front Immunol. 2023:14:1152457. 12. Yu M, et al. Hum Gene Ther. 2023;34(5-6):192-202. 13. Yang Q, et al. Int J Med Sci. 2021;18(8):1786-1797. 14. Zhang L, et al. Ann Med. 2021;53(1):1547-1559. 15. Cusatis R, et al. Transplant Cell Ther. 2024:S2666-6367(24)00482-2. 16. Leukemia & Lymphoma Society. Facts and Statistics Overview. Accessed January 2, 2025. https://www.lls.org/facts-and-statistics/facts-and-statistics-overview#Hodgkin%20(HL)%20and%20Non-Hodgkin%20(NHL)%20Lymphoma 17. Hoffman MS, et al. Transplant Cell Ther. 2023;29(7):440-448. 18. Dhaliwal S, et al. Cureus. 2024;16(5):e59951. 19. Nicolai CJ, et al. Blood. 2024;144(9):977-987. 20. Michels KR, et al. J Immunother Cancer. 2023;11(3):e006292. 21. Cambier C, et al. Journal for ImmunoTherapy of Cancer. 2023;11:doi: 10.1136/jitc-2023-SITC2023.0236. 22. O’Hara S, et al. Cancer Res. 2022;82(12_suppl):547.
A Powerful Therapeutic Approach
These core platforms work together in a complementary way. First, we apply VivoVec and the RACR/CAR payload architecture to stimulate the body to grow tumor-fighting T cells. We then deploy TumorTags to direct these tumor-fighting T cells to attack the tumor. The precision and control afforded by the integrated mechanisms of action of the platforms maximize the potential therapeutic effect while minimizing the risk of adverse events in the patient.
