A Brief History of the CAR T Cell Field
While chimeric antigen receptor (CAR) T cell therapies have revolutionized the treatment of blood-forming tissue cancers (i.e., hematologic malignancies), major limitations hinder their widespread application. Decades of CAR T cell therapy efforts, starting with “first generation” CAR T cell therapies in the 1990s and leading to the first CAR T cell therapies receiving FDA approval in 2017, have produced successful results in treating B cell malignancies, with long-term remission achieved in 30-40% of certain patient populations. Despite the promising clinical efficacy of CAR T cells in hematologic malignancies, significant challenges remain, including patient access, complex manufacturing, and high cost. Umoja Biopharma is focused on developing “off-the-shelf” cancer therapies to overcome these challenges.
Introducing Umoja Biopharma’s Engineered iPSC Platform
As part of Umoja Biopharma’s mission to deliver “off-the-shelf” therapies for cancer, we are developing an Engineered induced Pluripotent Stem Cells (iPSCs) platform. While the cell therapy industry has demonstrated the transformative potential of using gene-engineered, patient-derived cells for treating specific disease indications, many challenges with using patient-derived materials remain, including limited expansion capacity and scalability, manufacturing complexity, high cost, variability from patient to patient, and patient access. Umoja Biopharma’s Engineered iPSCs platform is designed to overcome these challenges by instead utilizing iPSCs. iPSCs are pluripotent stem cells, a type of cell theoretically capable of differentiating into any other cell type – including lymphocytes such as T cells and natural killer (NK) cells that are applicable to the treatment of cancer. Using iPSCs, we aim to enable scalable and simplified manufacturing of cancer-fighting cells like NK cells and T cells, thus reducing costs and improving patient access to cutting edge cellular cancer therapies.
iPSCs possess an unlimited expansion capacity, meaning they can reproduce and proliferate indefinitely, potentially generating a nearly endless supply of differentiated immune cells for cancer therapies. iPSCs are also amenable to precision multiplex genome editing, allowing introduction of multiple genetic modifications to enhance the cancer-fighting capabilities and safety of the immune cells they eventually become. iPSCs can similarly be engineered with the goal of protecting them against allogeneic rejection by the patient’s own immune system, improving both their initial expansion and duration of engraftment.
Furthermore, while either patient-derived or donor-derived blood materials are inconsistent, iPSCs provide a consistent starting material originating from a single cellular clone, which we believe will enable genomic consistency and integrity in the final cellular product. Taken together, we believe that our Engineered iPSCs platform offers solutions to many of the challenges of using blood-derived materials for the generation of cellular cancer therapies by providing an approach to create a precisely-edited, consistent, scalable cell therapy manufacturing process with reduced manufacturing complexity.
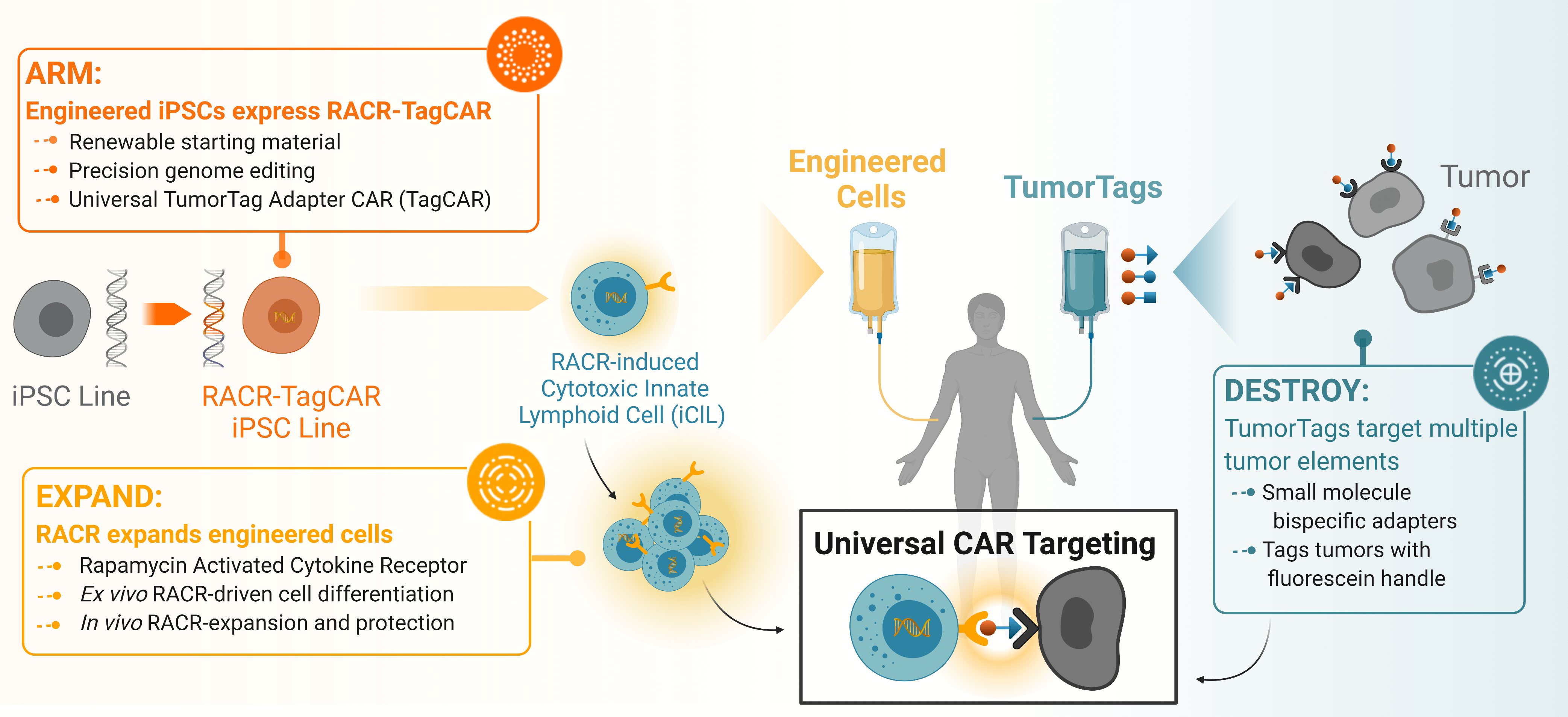
Overview of Umoja’s integrated platform detailing how the RACR-iCIL cells are engineered to express the Rapamycin Activated Cytokine Receptor to create the RACR-TagCAR iPSC line that can be induced by infusion of rapamycin to increase engraftment and persistence. These can be co-infused with TumorTag small molecules to universally label tumor and stromal tissue for precise targeting by RACR-CAR T cells.
Umoja Biopharma’s Technology Enables a Differentiated iPSC Therapy Platform
Umoja Biopharma’s engineered iPSCs are designed to provide an “off-the-shelf,” or ex vivo and allogeneic, immunotherapy treatment option by creating precisely engineered iPSCs, outside of patients, that express both our RACR cytokine receptor system and universal TagCAR.
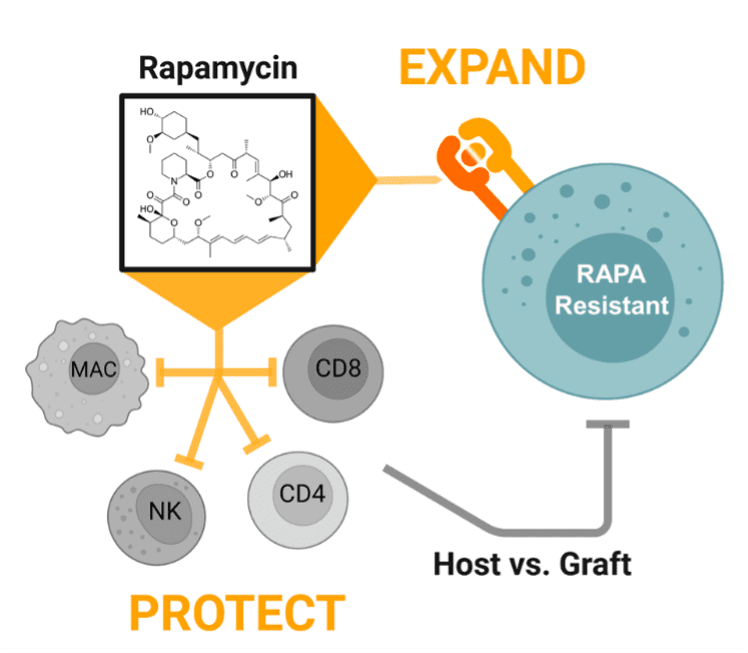
Rapamycin administration in patients who already have received infusions of RACR-iCIL cells have a multimodal effect: 1) promoting engraftment with a protective effect against host versus graft responses and, 2) promoting expansion through activation of cytokine signaling pathways.
Engraftment and persistence of therapeutic cells are key challenges in the cell and gene therapy space that are commonly achieved by treating a patient with highly toxic chemotherapy prior to administration of the cell therapy. Persistence is key to achieving durable tumor remission and has proven to be a challenge in the allogeneic cell therapy space in part due to the anti-allograft response against the therapeutic cells. To address the challenge of achieving sufficient persistence of therapeutic cells, Umoja Biopharma is developing a synthetic cytokine receptor system, the rapamycin-activated cytokine receptor, or RACR platform. The potential benefits of the RACR system are realized both in the manufacturing process and in the patient. During manufacturing, RACR activation is used to drive differentiation of cells with potent cancer killing function, and to eliminate the need to add expensive growth factors and other raw materials. Once these engineered cells, or iPSC-derived RACR-induced cytotoxic innate lymphoid (RACR-iCIL) cells, are administered to the patient, RACR activation can be used to support the engraftment, persistence, and effector functions of therapeutic cells.
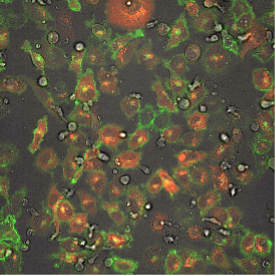
This fluorescence microscope image shows iPSC-derived NK cells engineered to produce Umoja Biopharma’s RACR and universal TagCAR (clear cells; brightfield) cultured together with breast cancer cells (red) tagged using TumorTag (green). To watch the NK cells in action – recognizing and destroying the cancer cells – see the video below.
The capacity of the RACR system to support therapeutic cell expansion and survival may allow therapeutic RACR-iCIL cells to be administered without the need for toxic lymphodepletion. RACR-iCIL cells are expected to expand in number in vivo when rapamycin, an FDA-approved drug, is administered to the patient. Rapamycin also has the native capacity to suppress the activity and expansion of immune cells that are not expressing the RACR system. Thus, treating patients with rapamycin is expected to not only bolster the survival and expansion of the therapeutic RACR-iCIL cells, but also inhibit immune responses which might target and limit the persistence of the therapeutic cells.
Once in a patient’s body, these engineered RACR-iCIL cells are designed to target and destroy tumor cells through a universal TagCAR that engages tumor cells labeled with Umoja Biopharma’s TumorTags. While a significant challenge in the immunotherapy field is unpredictable efficacy of solid tumor treatments due to hostile tumor microenvironments and tumor heterogeneity, our TumorTag platform aims to improve efficacy by labeling multiple targets in the tumor environment with a cocktail of bispecific small molecule adapters (TumorTags) that are recognized by our universal TagCAR.
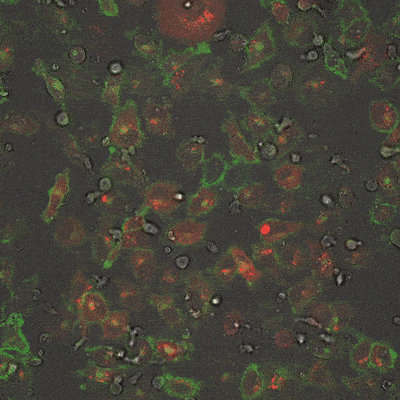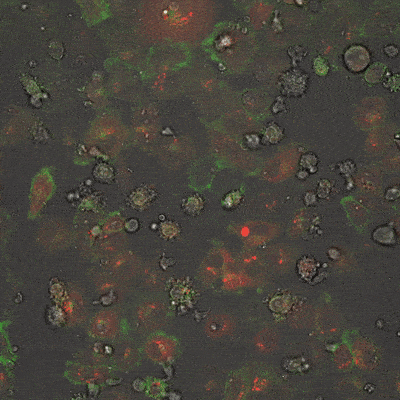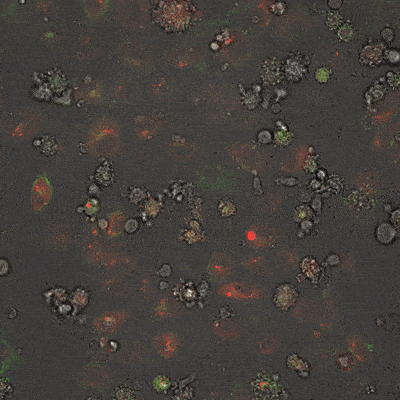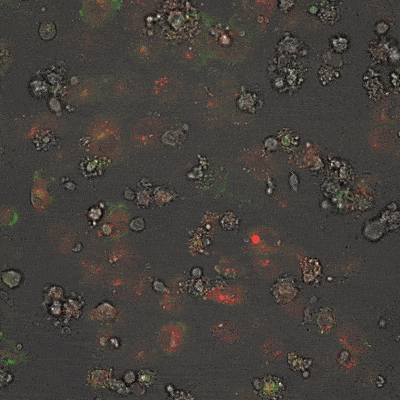
As shown in the video here, when RACR-iCIL cells (clear/brightfield) producing TagCAR are cultured with breast cancer cells (red) tagged via TumorTag (green), these RACR-iCIL cells can recognize and destroy the cancer cells.
Cautionary Note Regarding Forward-Looking Statements
This blog contains forward-looking statements about Umoja Biopharma, Inc. (the “Company,” “we,” “us,” or “our”). The Company has based these forward-looking statements largely on its current expectations, estimates, forecasts and projections about future events and financial trends that it believes may affect its financial condition, results of operations, business strategy and financial needs. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events. These statements are subject to risks and uncertainties that could cause the actual results to vary materially, including, among others, the risks inherent in drug development such as those associated with the initiation, cost, timing, progress and results of the Company’s current and future research and development programs, preclinical and clinical trials, as well as the economic, market and social disruptions due to the ongoing COVID-19 public health crisis. Except as required by law, the Company undertakes no obligation to update publicly any forward-looking statements for any reason.